Abstract
Background: Anti-CD38 monoclonal antibodies are used to treat newly diagnosed and relapsed or refractory multiple myeloma. Previous studies have shown an increased risk of infection while on daratumumab due to the immunosuppressive effect on malignant and normal plasma cells. We sought to retrospectively analyze and further understand the correlation of immunoglobin levels and risk of infection during treatment with anti-CD38 monoclonal antibodies in Multiple Myeloma.
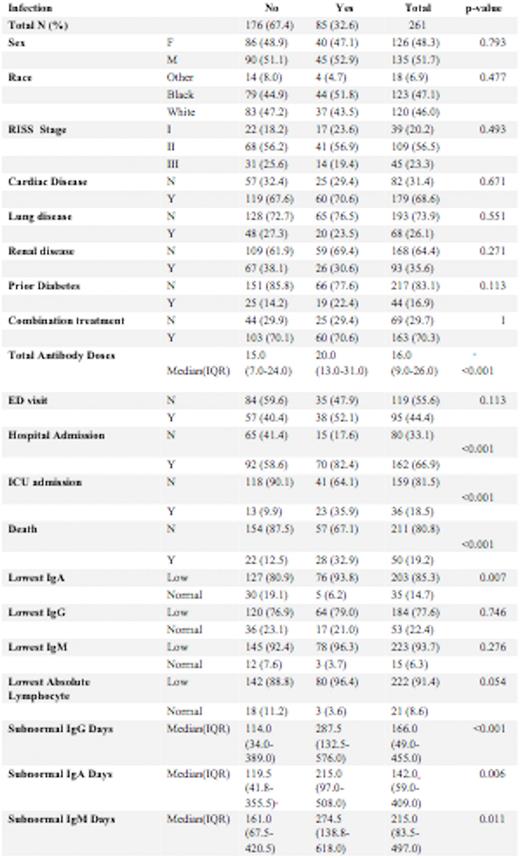
Methods: Analysis was performed on data from 261 patients who had received anti-CD38 monoclonal antibody therapy as part of treatment for multiple myeloma between November 11, 2016, and May 11, 2022. 51.7% were male and 48.3% were female. 47.1% of the patients identified as Black, 46% as White, and 6.9% as other races. 14.9% of the patients had Stage I, 41.8% had Stage II and 17.2% had Stage III disease, based on the Revised International Staging System (RISS). Baseline renal disease was present in 35.6% of patients. Median number of anti-CD38 Monoclonal antibody infusions was 16. We examined rates of specific infections (determined by ICD-10 codes), including pneumonia, urinary tract infection, bacteremia, and sepsis, as well as emergency room visits, hospitalizations including ICU stays for up to a year after completing treatment. These rates were correlated with immunoglobulin levels, absolute lymphocyte count, and the presence of concomitant treatment with a proteasome inhibitor or immunomodulator.
Results: The overall infection rate among patients receiving anti-CD 38 monoclonal antibody treatment was 32.6%. Bacterial infection developed in 22.2%, viral infections in 17.6%, and fungal infections in 3.4% of patients. SARS-CoV-2 infection rate was 13.4%. The median number of infusions was 15 in those without an infection and 20 in those who developed an infection (p<0.001).19.2% of patients who began anti-CD38 monoclonal antibody treatment during the study period have expired, of whom 56.0 % had an infection. Higher rates of infection were noted with lower IgA levels (p=0.007). There was no significant difference in infection rate with lower IgG and IgA levels or lower absolute lymphocyte levels (p=0.054). However, in patients with an infection there was a longer duration of sub-normal IgG days (114 vs 287 days, p=<0.001), IgA (119.5 vs 215, p=0.006), and IgM (161 vs 274.5, p=0.011). 44.4% of patients had an emergency room visit of whom 40.0% were due to infections (p=0.113). 66.9% of patents required hospital admissions of whom 43.2% had an infection) p<0.001). 18.5% of treated patients required ICU stays of whom 63.9% had an infection) p<0.001).
Discussion:Conclusions: A longer duration of subnormal Immunoglobulin levels correlated with higher risk of infection in Multiple Myeloma patients treated with anti-CD 38 monoclonal antibodies. Lowest Ig A levels during treatment correlated with a higher rate of infection while lowest IgG levels did not. Patients who developed infectious complications while on treatment with anti-CD 38 monoclonal antibodies had a significantly higher risk of hospitalization, ICU stays, and death. Prophylactic intravenous immunoglobulin can be studied to evaluate mitigation of infection in the highest risk subpopulation identified in this analysis such as those with prolonged subnormal Immunoglobulin levels.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal